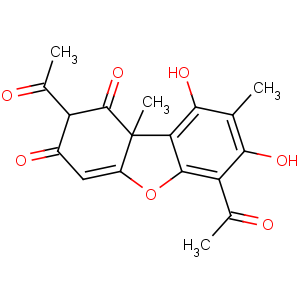
Title: Usnic Acid
CAS Registry Number: 125-46-2
CAS Name: 2,6-Diacetyl-7,9-dihydroxy-8,9b-dimethyl-1,3(2
H,9b
H)-dibenzofurandione
Synonyms: usninic acid; usnein
Molecular Formula: C18H16O7
Molecular Weight: 344.32
Percent Composition: C 62.79%, H 4.68%, O 32.53%
Literature References: Antibacterial substance found in lichens. Isoln from varieties of
Usnea barbata (L.) Wigg.,
Usneaceae: Rochleder, Heldt,
Ann. 48, 11 (1843); Widman,
Ann. 310, 230 (1900);
324, 139 (1902). Isoln from
Ramalina reticulata: Marshak,
Public Health Rep. 62, 3 (1947); Stark
et al., J. Am. Chem. Soc. 72, 1819 (1950). Occurs in nature in both the
d- and
l-forms as well as a racemic mixture. Structure: Curd, Robertson,
J. Chem. Soc. 1937, 894; Sch?pf, Ross,
Ann. 546, (1941); Barton, Brunn,
J. Chem. Soc. 1953, 603. Resolution of (±)-usnic acid: Dean
et al., ibid. 1250. Synthesis: Barton
et al., ibid. 1956, 530. Biosynthesis
in vitro: Penttila, Fales,
Chem. Commun. 1966, 656. Absolute configuration of (+)-form: S. Huneck
et al., Tetrahedron Lett. 22, 351 (1981). Toxicity study:
Antibiotics I, D. Gottlieb, P. Shaw, Eds. (Springer Verlag, New York, 1967) p 611.
Properties: Yellow orthorhombic prisms from acetone, mp 204°. [a]D16 +509.4° (c = 0.697 in chloroform). Monobasic acid. Soly at 25° (g/100 ml): water <0.01; acetone 0.77; ethyl acetate 0.88; ethanol 0.02; methyl Cellosolve 0.22; ethyl Cellosolve 0.32; furfural 7.32; furfuryl alcohol 1.21. LD50 i.v. in mice: 25 mg/kg (Gottlieb, Shaw).
Melting point: mp 204°
Optical Rotation: [a]D16 +509.4° (c = 0.697 in chloroform)
Toxicity data: LD50 i.v. in mice: 25 mg/kg (Gottlieb, Shaw)
Derivative Type: Sodium salt dihydrate
Molecular Formula: C18H15NaO7.2H2O
Molecular Weight: 402.33
Percent Composition: C 53.74%, H 4.76%, Na 5.71%, O 35.79%
Properties: Pale yellow, silky needles. Slightly sol in water.