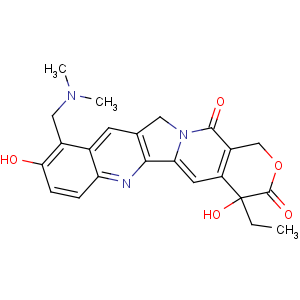
Title: Topotecan
CAS Registry Number: 123948-87-8
CAS Name: (4
S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1
H-pyrano[3¢,4¢:6,7]indolizino[1,2-
b]quinoline-3,14(4
H,12
H)-dione
Synonyms: 9-[(dimethylamino)methyl]-10-hydroxy-(20
S)-camptothecin; hycamptamine
Manufacturers' Codes: SKF-104864
Molecular Formula: C23H23N3O5
Molecular Weight: 421.45
Percent Composition: C 65.55%, H 5.50%, N 9.97%, O 18.98%
Literature References: DNA topoisomerase I inhibitor; semisynthetic analog of camptothecin,
q.v. Prepn: J. C. Boehm
et al., EP 321122;
eidem, US 5004758 (1989, 1991 both to SmithKline Beecham); W. D. Kingsbury
et al., J. Med. Chem. 34, 98 (1991). HPLC determn in plasma: J. H. Beijnen
et al., J. Pharm. Biomed. Anal. 8, 789 (1990). Clinical pharmacology: E. K. Rowinsky
et al., J. Clin. Oncol. 10, 647 (1992); and pharmacokinetics: L. J. C. van Warmerdam
et al., Cancer Chemother. Pharmacol. 38, 254 (1996). Clinical evaluation in ovarian cancer: A. P. Kudelka
et al., J. Clin. Oncol. 14, 1552 (1996); in small cell lung cancer: J. H. Schiller
et al., ibid. 2345. Review of clinical toxicity: K. Seiter,
Expert Opin. Drug Safety 4, 45-53 (2005).
Derivative Type: Hydrochloride
CAS Registry Number: 119413-54-6
Manufacturers' Codes: NSC-609669; SKF-104864A
Trademarks: Hycamtin (GSK)
Molecular Formula: C23H23N3O5.HCl
Molecular Weight: 457.91
Percent Composition: C 60.33%, H 5.28%, N 9.18%, O 17.47%, Cl 7.74%
Properties: Light yellow to greenish powder, mp 213-218° (dec). Soluble in water up to 1 mg/ml.
Melting point: mp 213-218° (dec)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkaloids/Natural Products; Camptothecin Derivatives; Topoisomerase I Inhibitor.