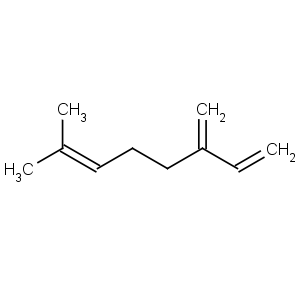
Title: b-Myrcene
CAS Registry Number: 123-35-3
CAS Name: 7-Methyl-3-methylene-1,6-octadiene
Synonyms: 2-methyl-6-methylene-2,7-octadiene
Molecular Formula: C10H16
Molecular Weight: 136.23
Percent Composition: C 88.16%, H 11.84%
Literature References: Found in oil of bay, verbena, hop, and others. Isoln: Power, Kleber,
Pharm. Rundsch. 13, 60 (1895);
see also Ruzicka, Stoll,
Helv. Chim. Acta 7, 272 (1924); Goulding, Roberts,
J. Chem. Soc. 105, 2614 (1914); Booker
et al., ibid. 1940, 1453; Kugler, Kováts,
Helv. Chim. Acta 46, 1480 (1963). Obtained by pyrolysis of b-pinene: Goldblatt, Palkin,
J. Am. Chem. Soc. 63, 3517 (1941); Rummelsburg,
US 2444790 (1948 to Hercules Powder); Houlihan
et al., J. Am. Chem. Soc. 81, 4692 (1959). Separation of isomers: Ohloff
et al., Ann. 675, 83 (1964). Synthesis: O. P. Vig
et al., Indian J. Chem. 7, 450 (1969);
11, 104 (1973);
13, 1244 (1975); T. Mandai
et al., Tetrahedron Lett. 22, 763 (1981). Formation from isoprene: K. Takabe
et al., Synthesis 1977, 307.
Properties: Oil. Pleasant odor. d420 0.794.
nD20 1.4709. uv max (ethanol): 226 nm (e 16,100). Practically insol in water. Sol in alcohol, chloroform, ether, glacial acetic acid.
Index of refraction: nD20 1.4709
Absorption maximum: uv max (ethanol): 226 nm (e 16,100)
Density: d420 0.794
Derivative Type: a-Myrcene
Synonyms: 2-Methyl-6-methylene-1,7-octadiene
Properties: bp10 44°.
nD25 1.4661. d2525 0.7959. uv max (isooctane): 224.5 nm (e 18,600). Not found in nature. Prepn: Mitzner
et al., J. Org. Chem. 30, 646 (1965); Vig
et al., J. Indian Chem. Soc. 50, 329 (1973).
Boiling point: bp10 44°
Index of refraction: nD25 1.4661
Absorption maximum: uv max (isooctane): 224.5 nm (e 18,600)
Density: d2525 0.7959
Use: b-Myrcene as an intermediate in the manuf of perfume chemicals.