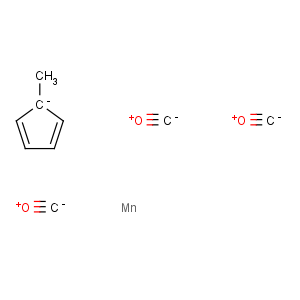
Title: MMT
CAS Registry Number: 12108-13-3
CAS Name: Tricarbonyl[(1,2,3,4,5-h)-1-methyl-2,4-cyclopentadien-1-yl]manganese
Synonyms: methylcyclopentadienylmanganese tricarbonyl; MCMT; tricarbonyl(h5-methylcyclopentadienyl)manganese(I); TCMn
Manufacturers' Codes: AK-33X
Molecular Formula: C9H7MnO3
Molecular Weight: 218.09
Percent Composition: C 49.56%, H 3.24%, Mn 25.19%, O 22.01%
Literature References: Organometallic antiknock compound used to increase the octane quality of fuel. Prepn: J. E. Brown
et al., US 2818417 (1957 to Ethyl Corp.). Evaluation of antiknock properties: R. J. Riggs
et al., Proc. 37th Natural Gas Assoc. Am. 1958, 51. Effect on auto emissions: W. R. Pierson
et al., J. Air Pollut. Control. Assoc. 28, 692 (1978). Use as dopant in Mn deposition: K. Hirabayashi, H. Kozawaguchi,
Jpn. J. Appl. Phys. 25, 711 (1986). Pyrolysis mechanism in deposition: S. Wen-bin
et al., J. Cryst. Growth 113, 1 (1991); I. M. T. Davidson
et al., J. Mater. Chem. 4, 13 (1994). GC determn in gasoline: W. A. Aue
et al., Anal. Chem. 62, 2453 (1990); and in air: V. S. Gaind
et al., Analyst 117, 161 (1992). Review as octane enhancer: J. D. Bailie,
Oil Gas J. 74, 69-72 (1976); D. P. Hollrah, A. M. Burns,
ibid. 89, 86-90 (1991). Review of toxicology: J. Stara
et al., Proc. 4th Ann. Conf Environment. Toxicol. AD-781 031, 251-270 (1973); P. J. Abbott,
Sci. Total Environ. 67, 247-255 (1987); and environmental assessment: D. R. Lynam
et al., ibid. 93, 107-114 (1990.)
Properties: Yellow orange liquid, fp -0.75°.
nD20 1.5873. d204 1.3942. Vapor pressure ranges from 8 mm at 100° to 360.6 mm at 200°. Readily sol in hydrocarbons and the usual organic solvents including hexane, alchohols, ether, acetone, ethylene glycol, lubricating oils, and hydrocarbon fuels, such as gasoline and diesel fuel. LD50 orally in mice, guinea pig, rabbit: 352; 905; 95 mg/kg; orally in male, female rats: 175 ±33; 89 ±14; LC50 inhalation in rat: 0.22 mg/l (Stara).
Index of refraction: nD20 1.5873
Density: d204 1.3942
Toxicity data: LD50 orally in mice, guinea pig, rabbit: 352; 905; 95 mg/kg; orally in male, female rats: 175 ±33; 89 ±14; LC50 inhalation in rat: 0.22 mg/l (Stara)
Use: Antiknock fuel additive; dopant for Mn.