Title: Chloranil
CAS Registry Number: 118-75-2
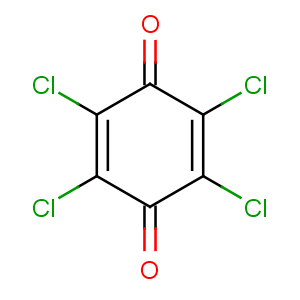
CAS Name: 2,3,5,6-Tetrachloro-2,5-cyclohexadiene-1,4-dione
Synonyms: 2,3,5,6-tetrachloro-
p-benzoquinone; tetrachloroquinone
Trademarks: Spergon; Vulklor
Molecular Formula: C6Cl4O2
Molecular Weight: 245.88
Percent Composition: C 29.31%, Cl 57.68%, O 13.01%
Literature References: Prepd from
p-phenylenediamine or phenol by treating with KClO3 and HCl. Because of its great resistance to further oxidation, chloranil is formed as the final product of the chlorate-HCl oxidation of many aromatic compds. For comprehensive list
see Huntress,
Organic Chlorine Compounds (New York, 1948). Laboratory procedure starting with phenol or
p-chlorophenol: Fierz-David, Blangey,
Grundlegende Operationen der Farbenchemie (Vienna, 5th ed., 1943) p 140. Use as reagent for pamaquine,
q.v., in urine: Schulemann
et al., Chem. Zentralbl. 1928, I, 2193. Aqueous decomposition study: D. H. Sarr
et al., Environ. Sci. Technol. 29, 2735 (1995).
Review: Health and Environmental Effects Profile for Chloranil (EPA PB88-219696, 1986) 59 pp.
Properties: Golden-yellow platelets from acetic acid or acetone. Monoclinic prisms from benzene or toluene or by sublimation
in vacuo, mp 290°. Absorption spectrum in chloroform: Lifschitz
et al., Rec. Trav. Chim. 43, 276, 658 (1924). Insol in water; almost insol in cold petr ether, cold alcohol. Sol in ether; sparingly sol in chloroform, carbon tetrachloride, carbon disulfide. Solubility data: Dimroth, Bamberger,
Ann. 438, 103, 106 (1924).
Melting point: mp 290°
CAUTION: Potential symptoms of overexposure are watery diarrhea, CNS depression, coma. Not absorbed percutaneously; direct contact may cause skin irritation.
See Clinical Toxicology of Commercial Products, R. E. Gosselin
et al., Eds. (Williams & Wilkins, Baltimore, 5th ed., 1984) p II-317.
Use: Agricultural fungicide, dye intermediate, reagent. Manuf of electrodes for pH measurement.