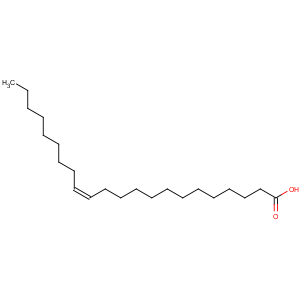
Title: Erucic Acid
CAS Registry Number: 112-86-7
CAS Name: (
Z)-13-Docosenoic acid
Synonyms: D13-
cis-docosenoic acid
Molecular Formula: C22H42O2
Molecular Weight: 338.57
Percent Composition: C 78.04%, H 12.50%, O 9.45%
Literature References: A monoethenoid acid found in the seed fats of
Cruciferae and
Tropaeolaceae. It constitutes 40 to 50% of the total fatty acids of rapeseed, mustard and wallflower seed, and it represents up to 80% of fatty acids of nasturtium seeds,
cf. K. S. Markley,
Fatty Acids Part I (Interscience, New York, 2nd ed., 1960) p 138-139. Prepn of a crude product by alkaline hydrolysis of rapeseed oil: Noller, Talbot,
Org. Synth. coll. vol. II, 258 (1943). A purer product is obtained by the method of Dorée and Pepper,
J. Chem. Soc. 1942, 477, which involves a fractional precipitation and crystallization. Prepn of a pure product by acid soap crystallization: Chobanov
et al., Chem. Ind. (London) 1965, 606. Synthesis: Bowman,
J. Chem. Soc. 1950, 177, 325; Bounds
et al., ibid. 1953, 2393. Treatment with nitric acid yields the
trans isomer, brassidic acid,
q.v.: Dorée, Pepper,
loc. cit. Brief review: E. Lower,
Manuf. Chem. 56(6), 61-63 (1985).
Properties: Needles from alcohol, mp 33.8°. Iodine value 74.98; neutralization value 165.72. d455 0.860. bp760 381.5° (decompn); bp400 358.8°; bp100 314.4°; bp60 300.2°; bp20 270.6°; bp5 239.7°; bp1.0 206.7°.
nD45 1.4534;
nD65 1.44794. Insol in water. About 175 g dissolve in 100 ml ethanol and about 160 g dissolve in 100 ml methanol. Very sol in ether.
Melting point: mp 33.8°
Boiling point: bp760 381.5° (decompn); bp400 358.8°; bp100 314.4°; bp60 300.2°; bp20 270.6°; bp5 239.7°; bp1.0 206.7°
Index of refraction: nD45 1.4534;
nD65 1.44794
Density: d455 0.860