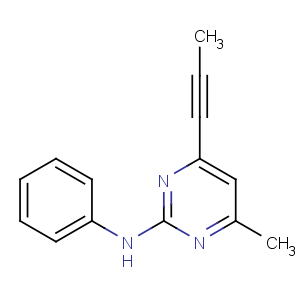
Title: Mepanipyrim
CAS Registry Number: 110235-47-7
CAS Name: 4-Methyl-
N-phenyl-6-(1-propynyl)-2-pyrimidinamine
Synonyms: 2-anilino-4-methyl-6-(1-propynyl)pyrimidine;
N-(4-methyl-6-prop-1-ynylpyrimidin-2-yl)aniline
Manufacturers' Codes: KIF-3535; KUF-6201
Trademarks: Frupica (Kumiai)
Molecular Formula: C14H13N3
Molecular Weight: 223.27
Percent Composition: C 75.31%, H 5.87%, N 18.82%
Literature References: Antifungal for use in food crops; inhibits secretion of host-cell wall degrading enzymes. Prepn: S. Ito
et al., EP 224339;
eidem, US 4814338 (1987, 1989 both to Kumiai; Ihara); and biological activity: S. Hayashi
et al., J. Pestic. Sci. 22, 165 (1997). Fungicidal activity and field trials: S. Maeno
et al., Brighton Crop Prot. Conf. - Pests Dis. 1990, 415. Mode of action study: I. Miura
et al., Pestic. Biochem. Physiol. 48, 222 (1994). GC-MS determn in grapes, must and wine: P. Cabras
et al., J. AOAC Int. 81, 1185 (1998). Metabolism in tomato seedlings: M. Ikeda
et al., J. Pestic. Sci. 23, 9 (1998).
Properties: White solid with two crystalline modifications. mp 125-126° (Maeno); also reported as 132.8° (Hayashi). Specific gravity 1.2025. Soly in water at 20°: 5.58 mg/l. Sol in most organic solvents. Vapor pressure at 20°: 1.03 ′ 10-5 torr. Log P (octanol/water): 3.42. LD50 in mice, rats, bobwhite, mallard (mg/kg): >5000, >5000, >2250, >2250 orally; in rats (mg/kg): >2000 dermally. LC50 in bluegill, rainbow trout (mg/l): 3.8, 3.1 (Maeno).
Melting point: mp 125-126° (Maeno); also reported as 132.8° (Hayashi)
Log P: Log P (octanol/water): 3.42
Toxicity data: LD50 in mice, rats, bobwhite, mallard (mg/kg): >5000, >5000, >2250, >2250 orally; in rats (mg/kg): >2000 dermally; LC50 in bluegill, rainbow trout (mg/l): 3.8, 3.1 (Maeno)
Use: Agricultural fungicide.