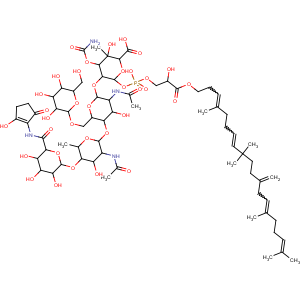
Title: Bambermycins
CAS Registry Number: 11015-37-5
Synonyms: Moenomycin; flavophospholipol
Trademarks: Flavomycin (Hoechst)
Literature References: Antibiotic complex comprised of at least 4 active components, moenomycins A, B1, B2 and C, with
moenomycin A being the major component. Obtained from cultures of
Streptomyces bambergiensis, S. ghanaensis, S. ederensis, S. geysiriensis and related strains: Wallh?user
et al., Antimicrob. Agents Chemother. 1965, 734
sqq.; NL 6602132 C.A. 66, 74946x (1967) and Lindner, Wallh?user,
US 3674866 (1966, 1972 both to Hoechst). Structural studies: Tschesche
et al., Ann. 720, 58 (1968);
Tetrahedron Lett. 1968, 2905;
1969, 141; Huber,
J. Antibiot. 25, 1226 (1972); P. Welzel
et al., Tetrahedron Lett. 1973, 227; F. J. Witteler
et al., ibid. 1979, 3493; P. Welzel
et al., Tetrahedron 39, 1583 (1983). Structure of moenomycin A: P. Welzel
et al., Angew. Chem. Int. Ed. 20, 121 (1981). Synthesis of
moenocinol, the lipid moiety: Tschesche, Reden,
Ann. 1974, 853; P. J. Kocienski,
J. Org. Chem. 45, 2037 (1980); R. M. Coates, M. W. Johnson,
ibid. 2685.
Review: G. Huber in
Antibiotics vol. 5, pt. 1, F. E. Hahn, Ed. (Springer-Verlag, New York, 1979) pp 135-153.
Properties: Complex is a colorless, amorphous solid, no definite mp, decompn starting at 200°C. uv max (water pH 7): 258 nm (E1%1cm 60). Sol in water, methanol, DMF; less sol in ethanol, propanol; slightly sol in ether, ethyl acetate. Insol in benzene, chloroform. Stable in neutral aq and methanolic solns; slowly decomp in acid and alkaline solns. LD50 in mice (mg/kg): >2000 orally, s.c. and i.p.; 1400 i.v. (Lindner, Wallh?user).
Absorption maximum: uv max (water pH 7): 258 nm (E1%1cm 60)
Toxicity data: LD50 in mice (mg/kg): >2000 orally, s.c. and i.p.; 1400 i.v. (Lindner, Wallh?user)
Therap-Cat: Antibacterial.
Therap-Cat-Vet: Antibacterial; feed additive (poultry, swine and calves).
Keywords: Antibacterial (Antibiotics); Aminoglycosides.