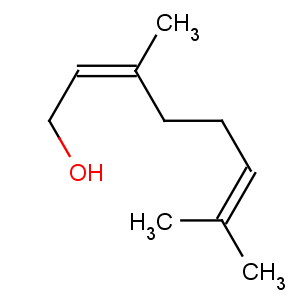
Title: Nerol
CAS Registry Number: 106-25-2
CAS Name: (Z)-3,7-Dimethyl-2,6-octadien-1-ol
Synonyms: cis-2,6-dimethyl-2,6-octadien-8-ol
Molecular Formula: C10H18O
Molecular Weight: 154.25
Percent Composition: C 77.87%, H 11.76%, O 10.37%
Literature References: The
cis-isomer of geraniol,
q.v.; found in many essential oils. Readily loses water and cyclizes forming dipentene. Isoln from neroli oil: Hesse, Zeitschel,
J. Prakt. Chem. 66, 502 (1902). Structure: Verley,
Bull. Soc. Chim. Fr. 25, 68 (1919); J. L. Simonsen,
The Terpenes vol. I (University Press, Cambridge, 2nd ed., 1947) pp 52-54. Stereochemistry: Burrell
et al., Proc. Chem. Soc. London 1959, 263; Bates
et al., J. Org. Chem. 28, 1086 (1963). Synthesis: Yukawa
et al., Bull. Chem. Soc. Jpn. 37, 158 (1964). Stereochemistry and synthesis: Burrell
et al., J. Chem. Soc. C 1966, 2144; K. Takabe
et al., Chem. Lett. 1977, 1025.
Properties: Liquid. Odor of sweet rose. bp745 224-225°; bp25 125°. d15 0.8813. Optically inactive. uv max: 189-194 nm (e 18000). Sol in abs alc.
Boiling point: bp745 224-225°; bp25 125°
Absorption maximum: uv max: 189-194 nm (e 18000)
Density: d15 0.8813
Derivative Type: Tetrabromide
Molecular Formula: C10H18Br4O
Molecular Weight: 473.87
Percent Composition: C 25.35%, H 3.83%, Br 67.45%, O 3.38%
Properties: Crystals, mp 118°.
Melting point: mp 118°
Derivative Type: Allophanate
Molecular Formula: C12H20N2O3
Molecular Weight: 240.30
Percent Composition: C 59.98%, H 8.39%, N 11.66%, O 19.97%
Properties: Needles from petr ether, mp 84-86°.
Melting point: mp 84-86°
Use: Base for manuf of perfumes.