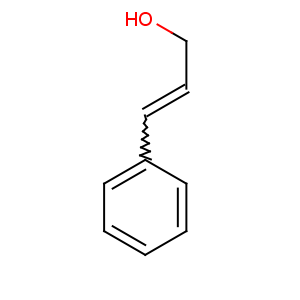
Title: Cinnamyl Alcohol
CAS Registry Number: 104-54-1
CAS Name: 3-Phenyl-2-propen-1-ol
Synonyms: cinnamic alcohol; styryl carbinol; g-phenylallyl alcohol
Molecular Formula: C9H10O
Molecular Weight: 134.18
Percent Composition: C 80.56%, H 7.51%, O 11.92%
Literature References: The
trans-form occurs (in the esterified form) in storax and in balsam Peru, cinnamon leaves, hyacinth oil. Obtained by the alkaline hydrolysis of storax. Prepd synthetically by reducing cinnamal diacetate with iron filings and acetic acid; from cinnamaldehyde by Meerwein-Ponndorf reduction with aluminum isopropoxide: Meerwein, Schmidt,
Ann. 444, 221 (1925). Review of risk assessment as fragrance ingredient: D. Bickers
et al., Food Chem. Toxicol. 43, 799-836 (2005); of toxicology: C. S. Letizia
et al., ibid. 837-866.
Properties: Needles or cryst mass. Odor of hyacinth. mp 30°. d3535 1.0397. bp 258°.
nD20 1.58190;
nD33 1.57580. Flash point, closed cup: >93.3°C. When small amounts of impurities are present as in the natural article (cinnamyl alcohol from storax), it remains fluid at lower temps than the melting point. Minimum congealing points specified by the Essential Oil Assn are: 33.0° for cinnamic alcohol pure; 28.0° for cinnamic alcohol prime; 20.0° for cinnamic alcohol from storax. Is oxidized slowly on exposure to heat, light and air. Sol in water, glycerol. Clearly sol in 3 vols 50% alc. Freely sol in alc, ether, other common organic solvents. LD50 (g/kg): 2.0 orally in rats; >5.0 dermally in rabbits (Letizia).
Melting point: mp 30°
Boiling point: bp 258°
Flash point: Flash point, closed cup: >93.3°C
Index of refraction: nD20 1.58190;
nD33 1.57580
Density: d3535 1.0397
Toxicity data: LD50 (g/kg): 2.0 orally in rats; >5.0 dermally in rabbits (Letizia)
Use: In perfumery; as deodorant in 12.5% soln in glycerol.