Title: Hydrazine Sulfate
CAS Registry Number: 10034-93-2
Synonyms: Hydrazinium sulfate; hydrazonium sulfate
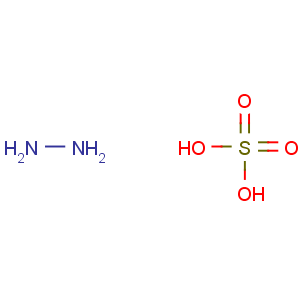
Molecular Formula: H6N2O4S
Molecular Weight: 130.12
Percent Composition: H 4.65%, N 21.53%, O 49.18%, S 24.64%
Line Formula: H2NNH2.H2SO4
Literature References: Prepd by Raschig synthesis: 2NH3.aq + [Ca(OCl)2/Na2CO3/colloid] and treatment with H2SO4. Starch, glue, or gelatin are used as colloids, and sodium hypochlorite may be used instead of bleaching powder: Adams, Brown,
Org. Synth. 2, 37 (1922); Audrieth, Nickles,
Inorg. Synth. 1, 90 (1939). Industrial prepn by the action of sodium hypochlorite on urea in the presence of NaOH:
BIOS Final Report 369; Moncrieff,
Manuf. Chem. 18, 177 (1947). Revised lab procedures: Pfeiffer, Simons,
Ber. 80, 127 (1947); Adams, Brown,
Org. Synth. coll. vol. I, 2nd ed. (1941), p 309. Crystal structure: Nitta
et al., Acta Crystallogr. 4, 289 (1951); J?nsson, Hamilton,
ibid. 26B, 536 (1970). Review of activity and clinical studies in cancer cachexia: J. Gold,
Nutr. Cancer 9, 59-66 (l987).
Properties: Orthorhombic crystals. Glass-like plates or prisms. d 1.378: Curtis, Jay,
J. Prakt. Chem. 39, 39 (1889); d7 2.016. mp 254°. Sol in about 33 parts water; freely sol in hot water. Insol in alcohol. pH of 0.2 molar aq soln 1.3.
Melting point: mp 254°
Density: d 1.378: Curtis, Jay,
J. Prakt. Chem. 39, 39 (1889); d7 2.016
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-145.
Use: In the gravimetric estimation of nickel, cobalt and cadmium; in the refining of rare metals; as antioxidant in soldering flux for light metals; as reducing agent in the analysis of minerals and slags; in separating polonium from tellurium; in tests for blood; for destroying fungi and molds; in the prepn of hydrazine hydrate.